I find that mineral identification is not a strong point of a lot of people here, and that questions are being asked in many places and it would be good to bring them together under one heading, that I will try to encourage here - I find that as a geologist fairly strong on mineralogy, I am repeatedly giving the same answers to different people in different places. Unfortunately there are already two similar headings under this section, Gemstones and Minerals, and Gemstones, Minerals and Fossils. and these questions are also often also asked under gold headings as well, so I will stick to this one, Gemstones and Minerals). I would suggest two headings, this one called "Series on identifying minerals" and another "Your mineral identification questions", in the hope that people might bring their questions together in one place with time. The idea with "Series on identifying minerals" is that I would get the ball rolling with a few basic blogs on how we do it, simple field equipment that is cheap and fits in your pocket that needed to do it, and then each of the important mineral properties, one by one, used in identification. The idea with "Your mineral identification questions" is that most questions be asked here where people know where to come, such as myself, other geos and those with experience in identification, to find what people want to know. Hopefully once the ball is rolling these might take on a life of their own, since I don't have time to do more than try to start this and look in occassionally - I also have a crust to earn.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Series on identifying minerals
- Thread starter user 4386
- Start date

Help Support Prospecting Australia:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
Good idea goldierocks. :Y: .. At the moment, things are here there and everywhere.... So hopefully things will sort itself out... 
May-be the Mods could make these headings a 'sticky'..
LW....
May-be the Mods could make these headings a 'sticky'..
LW....
This is now a sticky, thanks Goldirocks. :Y:
Series on identifying minerals - part 1
The most commonly used properties of minerals that geologists use, mostly in the field carrying only things in their day pack, are:
(1) External appearance (e.g. crystal shapes, parallel striations on crystal surfaces, the angles between adjacent crystal faces, twinning - which needs separate description)
(2) Specific gravity (i.e. density - two different minerals of the same size will commonly have a different weight , i.e. mass - they are "heavy" or "light" etc)
(3) Hardness (when scratched with different things that themselves have different hardness (e.g. does a "gold" coin scratch the mineral or does it scratch a "gold" coin), or get some idea by using a steel knife blade but using varying pressure to make a scratch (easy to make a deep scratch etc.)
(4) Fracture - when you break it, does it break along parallel surfaces, or like glass with a curved surface, or just rough like a clod of earth.
(5) Cleavage - these are planar surfaces that are not crystal faces (e.g. if you crush galena, which is lead sulphide, it will commonly break into cubes, mica - like white mica in a bread toaster, will commonly peel off into parallel sheets, asbestos will break into smaller and smaller parallel hair-like fibres (which makes them dangerous in your lungs, where the ends of the fibres dig into the lung surface). These things are breaking along cleavage planes or surfaces, which are sometimes parallel to crystal faces (which are not often present in your specimen), or they have a constant angular relationship to such (potential) crystal faces.
(6) Other physical properties. Flexibility - some of the micas will snap back to their original shape if you bend a plate of mica then release it (they are ELASTIC) - others will simply stay in the new shape you have bent them to (they are simply FLEXIBLE but not elastic, more plastic). BRITTLE minerals make a cracking sound when you hit them with a hammer, and crumble into small fragments (e.g. quartz), softer minerals like talc, or graphite simply quietly turn to fine powder. DUCTILE(sometimes termed MALLEABLE) minerals such as gold or native copper can be hammered into flat sheets without forming fragments or powder, Strictly, ductile means they can be pulled out into long wires, malleable that they can be flattened, but they are related properties.
(7) Colour - this is colourless for some minerals, a characteristic and constant colour for others (sulphur is always yellow), or can have a limited range of colours due to impurity elements in the crystal (corundum can be red = ruby, blue = sapphire etc) - for example, the mineral beryl has a green gem variety called emerald caused by chromium traces in the beryl. Or colours can be everything under the sun (precious opal, caused by light refracting through the different diameter spheres that comprise it, like sunlight travelling through rain to give a rainbow). Some minerals appear different colours under different types of light, some change colour as you rotate them. Numerous minerals TARNISH at the surface due to chemical reaction with water in the air, so you should always break a fresh surface to see the true colour (an example of tarnish is the mineral bornite, which has a constant reddish hue when fresh, but develops a beautiful surface tarnish ("peacock ore"). Some minerals change colour during prolonged exposure to sunlight (why silver cutlery must be polished regularly - and don't leave your emeralds in the sun!)
(8) Streak - the colour of the powdered mineral (scratch with a pocket-knife point. or scratch on a broken surface of porcelain. This is constant, regardless of colour variations in a mineral. The streak of white minerals is always white but that of coloured minerals (pyrite, chalcopyrite, hematite) can be quite different to the colour of the solid mineral (the streak of pyrite is greenish-black).
(9) Lustre. You know that something is metallic by looking at it, or glassy, or earthy, greasy, dull or pearly or brilliant like a diamond (we call that adamantine) - these are the lustres of minerals and are related to how light reflects off their surface. So metallic, non-metallic, sub-metallic, and all these variants - all have reasons (pearly lustre occurs in layered minerals, silky lustre in fibrous minerals).
(10) Transparency - transparent if writing can be read through it, translucent if light can shine through it, opaque if it blocks all light (this can be a function of how thick your sample is - we refer to thin fragments)
(11) Other optical properties like luminescence (under ultraviolet light), e.g. scheelite - or thermoluminescence (after heating as with some fluorite) - or triboluminescence (after crushing to powder or rubbing).
(12) Heat conductivity, e.g. native copper feels colder than amber because it conducts heat away more readily from your hand (an experienced genmstone polisher can tell coloured glass in this way by touching it against their cheek, which is sensitive to heat).
(13) Other physiological properties than heat are things related to "feel" - "greasy and smooth" graphite or talc, "dry and rough" chalk and kaolin (which are strictly rocks formed of the minerals calcite and clays).
(14) Taste (be wary) - halite or common salt tastes salty. other minerals bitter or sour (I do not do it usually but can under known circumstances e.g. if working in a salt mine)
(11) Magnetism - magnetic, like magnetite, or weakly magnetic like most pyrrhotite, or non-magnetic like pyrite or galena.
(12) Chemical properties - e.g. calcite reacts vigorously with a drop of cold, weak hydrochloric acid, another carbonate mineral like dolomite reacts weakly with the acid, whereas one like magnesite will not react at all. Dropped in a container of acid, calcite will rapidly dissolve, dolomite will take ages and leavea residue (unless the mixture is heated), magnesite will be sitting there a week later.
(13) There are of course expensive laboratory tests - but they are highly diagnostic (chemical analysis that determines the chemical elements present, XRay analysis that determines the internal crysal structure).
Three things:
(a) determine as many of these things as you can to tell a person who you want to identify a mineral for you - it is frustrating to have to always cross-examine and ask - What colour is it? Is it hard? What is its streak? Is it magnetic? as anyone can determine these most common properties (we don't mind asking non-mineralogists, but it helps us a lot).
(b) get a good cheap book with colour photos in it, that lists these properties of different minerals. I consider that the book "A field guide in color to Minerals, Rocks and Precious Stones by Bauer (publisher Chartwell Books Inc) is as good as any I know, it is small but is 207 pages of tables with 576 colour photos. I got mine new on a sale for $23 but there are plenty of similar books. It also discusses these propereties in more detail, how to determine them, field equipment etc.
(c) try to know the difference in naming between minerals, gems, rocks and industrial names - it will limit your confusion (because it is confusing). There are official names for mineral families (e.g. the amphiboles) and the individual minerals that comprise those families, or for particular shapes of those minerals (e.g. the amphiboles include hornblende and riebeckite - or riebeckites' fibrous variety which has a special mineral name, crocidolite, or an industrial name - blue asbestos). The industrial name white asbestos is used by industry for the fibrous mineral chrysotile which is simply a fibrous form of the mineral family serpentine. So "asbestos" is not a single mineral but the industrial name for quite diffferent unrelated minerals simply because they are all finely fibrous and heat and chemical resistant. Others asbestos includes the fibrous forms of the mineral tremolite that is part of the mineral family pyroxene.
Gems are simply attractive forms of minerals - ruby is the gem name for the red and transparent form of the mineral corundum. Emerald similarly for green beryl (or aquamarine if greenish blue).
Rocks are NOT minerals but are aggregates of minerals that occur as large bodies of material - so granite is a rock made of quartz, mica and feldspar, limestone is a rock made dominantly of grains of carbonate minerals such as calcite or dolomite - chert is a rock made of quartz, chalcedony, opal or combinations of these. To explain more simply - mineral families and their minerals are tightly defined by their chemical compositions and mineral strucure - these are constant. Gemmologists give attractive, coloured examples of these minerals gem names. Mineral companies give the minerals their own pet names, which will sometimes be the same name for quite different minerals (e.g kaolin is an industrial name for white clay, it may or may not be dominantly the clay mineral kaolinite). Rocks are composed of multiple minerals, or are large bodies of billions of grains of varying grain size of what can be a single mineral. For example, sandstone, quartzite, chert, flint might all consist dominantly of the mineral quartz. In sandstones you will see the grains with your eye, the same with quartzite except it is commonly very pure quartz and with grains strongly bound together, you won't see the grains in chert and chalcedony even under a hand lens - flint commonly occurs as rounded bodies tens of centimetres in diametre enclised by chalk.
It takes years to learn it all, but very little time to understand the different ways in which things are named - minerals and their families, gems, industrial materials and rocks.
The most commonly used properties of minerals that geologists use, mostly in the field carrying only things in their day pack, are:
(1) External appearance (e.g. crystal shapes, parallel striations on crystal surfaces, the angles between adjacent crystal faces, twinning - which needs separate description)
(2) Specific gravity (i.e. density - two different minerals of the same size will commonly have a different weight , i.e. mass - they are "heavy" or "light" etc)
(3) Hardness (when scratched with different things that themselves have different hardness (e.g. does a "gold" coin scratch the mineral or does it scratch a "gold" coin), or get some idea by using a steel knife blade but using varying pressure to make a scratch (easy to make a deep scratch etc.)
(4) Fracture - when you break it, does it break along parallel surfaces, or like glass with a curved surface, or just rough like a clod of earth.
(5) Cleavage - these are planar surfaces that are not crystal faces (e.g. if you crush galena, which is lead sulphide, it will commonly break into cubes, mica - like white mica in a bread toaster, will commonly peel off into parallel sheets, asbestos will break into smaller and smaller parallel hair-like fibres (which makes them dangerous in your lungs, where the ends of the fibres dig into the lung surface). These things are breaking along cleavage planes or surfaces, which are sometimes parallel to crystal faces (which are not often present in your specimen), or they have a constant angular relationship to such (potential) crystal faces.
(6) Other physical properties. Flexibility - some of the micas will snap back to their original shape if you bend a plate of mica then release it (they are ELASTIC) - others will simply stay in the new shape you have bent them to (they are simply FLEXIBLE but not elastic, more plastic). BRITTLE minerals make a cracking sound when you hit them with a hammer, and crumble into small fragments (e.g. quartz), softer minerals like talc, or graphite simply quietly turn to fine powder. DUCTILE(sometimes termed MALLEABLE) minerals such as gold or native copper can be hammered into flat sheets without forming fragments or powder, Strictly, ductile means they can be pulled out into long wires, malleable that they can be flattened, but they are related properties.
(7) Colour - this is colourless for some minerals, a characteristic and constant colour for others (sulphur is always yellow), or can have a limited range of colours due to impurity elements in the crystal (corundum can be red = ruby, blue = sapphire etc) - for example, the mineral beryl has a green gem variety called emerald caused by chromium traces in the beryl. Or colours can be everything under the sun (precious opal, caused by light refracting through the different diameter spheres that comprise it, like sunlight travelling through rain to give a rainbow). Some minerals appear different colours under different types of light, some change colour as you rotate them. Numerous minerals TARNISH at the surface due to chemical reaction with water in the air, so you should always break a fresh surface to see the true colour (an example of tarnish is the mineral bornite, which has a constant reddish hue when fresh, but develops a beautiful surface tarnish ("peacock ore"). Some minerals change colour during prolonged exposure to sunlight (why silver cutlery must be polished regularly - and don't leave your emeralds in the sun!)
(8) Streak - the colour of the powdered mineral (scratch with a pocket-knife point. or scratch on a broken surface of porcelain. This is constant, regardless of colour variations in a mineral. The streak of white minerals is always white but that of coloured minerals (pyrite, chalcopyrite, hematite) can be quite different to the colour of the solid mineral (the streak of pyrite is greenish-black).
(9) Lustre. You know that something is metallic by looking at it, or glassy, or earthy, greasy, dull or pearly or brilliant like a diamond (we call that adamantine) - these are the lustres of minerals and are related to how light reflects off their surface. So metallic, non-metallic, sub-metallic, and all these variants - all have reasons (pearly lustre occurs in layered minerals, silky lustre in fibrous minerals).
(10) Transparency - transparent if writing can be read through it, translucent if light can shine through it, opaque if it blocks all light (this can be a function of how thick your sample is - we refer to thin fragments)
(11) Other optical properties like luminescence (under ultraviolet light), e.g. scheelite - or thermoluminescence (after heating as with some fluorite) - or triboluminescence (after crushing to powder or rubbing).
(12) Heat conductivity, e.g. native copper feels colder than amber because it conducts heat away more readily from your hand (an experienced genmstone polisher can tell coloured glass in this way by touching it against their cheek, which is sensitive to heat).
(13) Other physiological properties than heat are things related to "feel" - "greasy and smooth" graphite or talc, "dry and rough" chalk and kaolin (which are strictly rocks formed of the minerals calcite and clays).
(14) Taste (be wary) - halite or common salt tastes salty. other minerals bitter or sour (I do not do it usually but can under known circumstances e.g. if working in a salt mine)
(11) Magnetism - magnetic, like magnetite, or weakly magnetic like most pyrrhotite, or non-magnetic like pyrite or galena.
(12) Chemical properties - e.g. calcite reacts vigorously with a drop of cold, weak hydrochloric acid, another carbonate mineral like dolomite reacts weakly with the acid, whereas one like magnesite will not react at all. Dropped in a container of acid, calcite will rapidly dissolve, dolomite will take ages and leavea residue (unless the mixture is heated), magnesite will be sitting there a week later.
(13) There are of course expensive laboratory tests - but they are highly diagnostic (chemical analysis that determines the chemical elements present, XRay analysis that determines the internal crysal structure).
Three things:
(a) determine as many of these things as you can to tell a person who you want to identify a mineral for you - it is frustrating to have to always cross-examine and ask - What colour is it? Is it hard? What is its streak? Is it magnetic? as anyone can determine these most common properties (we don't mind asking non-mineralogists, but it helps us a lot).
(b) get a good cheap book with colour photos in it, that lists these properties of different minerals. I consider that the book "A field guide in color to Minerals, Rocks and Precious Stones by Bauer (publisher Chartwell Books Inc) is as good as any I know, it is small but is 207 pages of tables with 576 colour photos. I got mine new on a sale for $23 but there are plenty of similar books. It also discusses these propereties in more detail, how to determine them, field equipment etc.
(c) try to know the difference in naming between minerals, gems, rocks and industrial names - it will limit your confusion (because it is confusing). There are official names for mineral families (e.g. the amphiboles) and the individual minerals that comprise those families, or for particular shapes of those minerals (e.g. the amphiboles include hornblende and riebeckite - or riebeckites' fibrous variety which has a special mineral name, crocidolite, or an industrial name - blue asbestos). The industrial name white asbestos is used by industry for the fibrous mineral chrysotile which is simply a fibrous form of the mineral family serpentine. So "asbestos" is not a single mineral but the industrial name for quite diffferent unrelated minerals simply because they are all finely fibrous and heat and chemical resistant. Others asbestos includes the fibrous forms of the mineral tremolite that is part of the mineral family pyroxene.
Gems are simply attractive forms of minerals - ruby is the gem name for the red and transparent form of the mineral corundum. Emerald similarly for green beryl (or aquamarine if greenish blue).
Rocks are NOT minerals but are aggregates of minerals that occur as large bodies of material - so granite is a rock made of quartz, mica and feldspar, limestone is a rock made dominantly of grains of carbonate minerals such as calcite or dolomite - chert is a rock made of quartz, chalcedony, opal or combinations of these. To explain more simply - mineral families and their minerals are tightly defined by their chemical compositions and mineral strucure - these are constant. Gemmologists give attractive, coloured examples of these minerals gem names. Mineral companies give the minerals their own pet names, which will sometimes be the same name for quite different minerals (e.g kaolin is an industrial name for white clay, it may or may not be dominantly the clay mineral kaolinite). Rocks are composed of multiple minerals, or are large bodies of billions of grains of varying grain size of what can be a single mineral. For example, sandstone, quartzite, chert, flint might all consist dominantly of the mineral quartz. In sandstones you will see the grains with your eye, the same with quartzite except it is commonly very pure quartz and with grains strongly bound together, you won't see the grains in chert and chalcedony even under a hand lens - flint commonly occurs as rounded bodies tens of centimetres in diametre enclised by chalk.
It takes years to learn it all, but very little time to understand the different ways in which things are named - minerals and their families, gems, industrial materials and rocks.
Series on identifying minerals - part 2 HARDNESS
You should test hardness on a nice clean surface of fresh mineral (if it is weathered or a bit oxidised or tarnished it will be softer). Note that in some minerals hardness varies in different directions (e.g. black tourmaline = schorl, has a different hardness parallel to the length of the crystals compared to at right angles to this - so also check that).
Mohs hardness scale:
This is what we use to designate a hardness number:
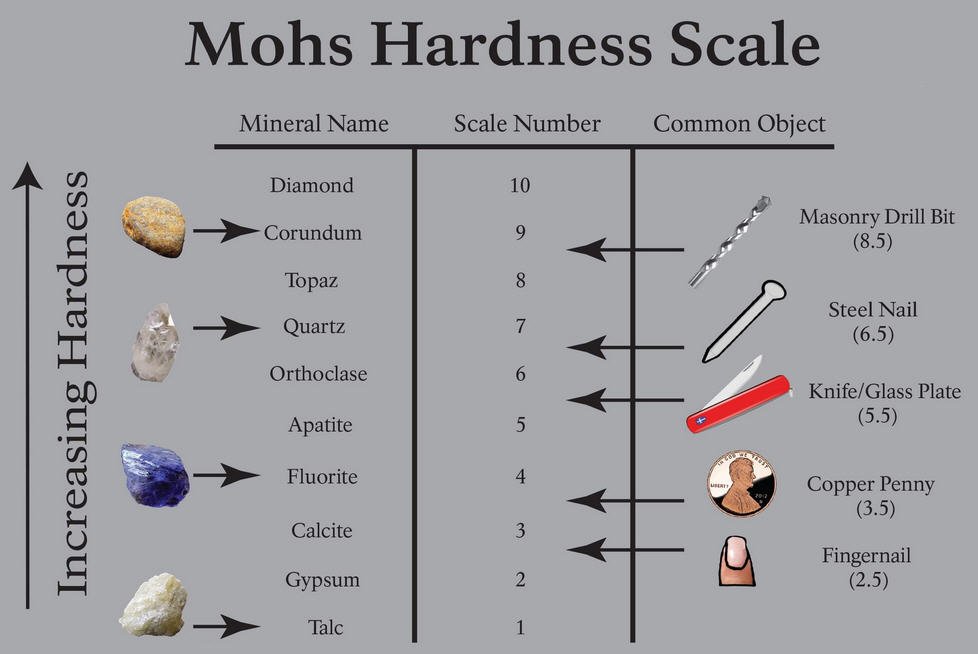 - sourced from advertisement for a kit at https://www.911metallurgist.com/blog/mohs-hardness-test-kit
- sourced from advertisement for a kit at https://www.911metallurgist.com/blog/mohs-hardness-test-kit
Note the first letter of each mineral on the hardness scale from 1 (softest) to 10 (hardest): TGCFAOQTCD. This is actually easy to memorise The Girls Can F[user-definable, e.g. flirt] And Other Queer Things Can Do
Tools:
1. special pocket-size kits with a different hardness point on each tool (e.g. a different mineral embedded in it) - can cost hundreds of dollars and I have never actually met anyone who forked out the money for this (available from companies like Prospectors Supplies), or
2. collect a few fragments of common minerals to make a kit to scratch with, or
3. What most of us do - you have fingernails and should have a "gold" coin, a very tiny pocketknife with sharp pointed steel blade (my entire knife is 4.5 cm long), a sharp fragment of quartz etc.That is enough to get a fair idea of hardness and costs nothing
Things made of steel vary a bit in hardness - so there is a case for using a bit of glass instead, or testing your knife blade etc with a known mineral. A copper penny and a $1 or $2 coin are fairly similar - the modern coins might be almost one half a number harder. So scratch it with quartz - it is softer than 7.
If you can scratch your unknown mineral with quartz it is softer than 7, with glass, it is softer than 5.5, can't scratch it with a coin but it scratches a coin, harder than 4. So your mineral probably has a hardness around 5.
A tiny pocket knife is a versatile tool since once you are confident of the hardness of your blade, you can use it to also scratch the mineral to get its "streak", and the fine point on the knife makes it easy to test hardness in different directions. By varying pressure with things softer than the blade you can tell if they are just a bit softer, or much softer, etc.
Get yourself a lanyard to hang around your neck - then your tools are always handy and difficult to lose. Get a pocket knife that is only a few cm long when closed and hang it from the lanyard. Buy a short bar magnet and hang it from the lanyard for testing magnetism (those sold at places like Prospectors Supplies are long like a ballpoint pen with a snib at top to hook over your pocket - these are OK but designed for putting in a breast pocket, where I lose them bending over regularly- since they are mostly plastic except the bottom less than a centimetre long magnet, I cut the length down and hang them from the lanyard. Get yourself a x10 and a x20 hand lens (magnifying glass that folds into a cover to keep it clean - not too large but best if optics are clear - places like Prospectors Supplies have them but you can Google these things). You need to attach things firmly to the lanyard (don't use really thin wire key-ring rings but sturdy ones that don't come open). Even lanyards need to be solid (and not metal of course because of the magnet and because metal lanyards easily snap when they catch on something and twist. I have found after years of testing that thick leather bootlaces make good lanyards, tying knots in the leather where things are attached using shorter bits of leather shoe-lace and using super-glue. If it catches on a bush the lanyard won't break and the itens won't easily snap off because they are also hanging from the lanyard from short bits of flexible leather (with robust small key-ring loop attachments on them). Make the lanyard long enough so that you can bring items on the lanyard like the hand lenses up to your eye comfortably, but not so long that it flops around and catches easily on bushes.
You should test hardness on a nice clean surface of fresh mineral (if it is weathered or a bit oxidised or tarnished it will be softer). Note that in some minerals hardness varies in different directions (e.g. black tourmaline = schorl, has a different hardness parallel to the length of the crystals compared to at right angles to this - so also check that).
Mohs hardness scale:
This is what we use to designate a hardness number:

Note the first letter of each mineral on the hardness scale from 1 (softest) to 10 (hardest): TGCFAOQTCD. This is actually easy to memorise The Girls Can F[user-definable, e.g. flirt] And Other Queer Things Can Do
Tools:
1. special pocket-size kits with a different hardness point on each tool (e.g. a different mineral embedded in it) - can cost hundreds of dollars and I have never actually met anyone who forked out the money for this (available from companies like Prospectors Supplies), or
2. collect a few fragments of common minerals to make a kit to scratch with, or
3. What most of us do - you have fingernails and should have a "gold" coin, a very tiny pocketknife with sharp pointed steel blade (my entire knife is 4.5 cm long), a sharp fragment of quartz etc.That is enough to get a fair idea of hardness and costs nothing
Things made of steel vary a bit in hardness - so there is a case for using a bit of glass instead, or testing your knife blade etc with a known mineral. A copper penny and a $1 or $2 coin are fairly similar - the modern coins might be almost one half a number harder. So scratch it with quartz - it is softer than 7.
If you can scratch your unknown mineral with quartz it is softer than 7, with glass, it is softer than 5.5, can't scratch it with a coin but it scratches a coin, harder than 4. So your mineral probably has a hardness around 5.
A tiny pocket knife is a versatile tool since once you are confident of the hardness of your blade, you can use it to also scratch the mineral to get its "streak", and the fine point on the knife makes it easy to test hardness in different directions. By varying pressure with things softer than the blade you can tell if they are just a bit softer, or much softer, etc.
Get yourself a lanyard to hang around your neck - then your tools are always handy and difficult to lose. Get a pocket knife that is only a few cm long when closed and hang it from the lanyard. Buy a short bar magnet and hang it from the lanyard for testing magnetism (those sold at places like Prospectors Supplies are long like a ballpoint pen with a snib at top to hook over your pocket - these are OK but designed for putting in a breast pocket, where I lose them bending over regularly- since they are mostly plastic except the bottom less than a centimetre long magnet, I cut the length down and hang them from the lanyard. Get yourself a x10 and a x20 hand lens (magnifying glass that folds into a cover to keep it clean - not too large but best if optics are clear - places like Prospectors Supplies have them but you can Google these things). You need to attach things firmly to the lanyard (don't use really thin wire key-ring rings but sturdy ones that don't come open). Even lanyards need to be solid (and not metal of course because of the magnet and because metal lanyards easily snap when they catch on something and twist. I have found after years of testing that thick leather bootlaces make good lanyards, tying knots in the leather where things are attached using shorter bits of leather shoe-lace and using super-glue. If it catches on a bush the lanyard won't break and the itens won't easily snap off because they are also hanging from the lanyard from short bits of flexible leather (with robust small key-ring loop attachments on them). Make the lanyard long enough so that you can bring items on the lanyard like the hand lenses up to your eye comfortably, but not so long that it flops around and catches easily on bushes.
Series on identifying minerals - part 3 VIEWING YOUR MINERAL
You ideally want a x10 and x20 hand lense as mentioned in part 2 - cheap plastic lenses readily scratch so glass is better, lenses that fold into a cover to keep dust off and scratches away. Not too large and clunky. Some have two lenses in one cover but I prefer two separate light and small hand lenses of x10 and x20.

This one is x10 with a focal length of 18 mm (most are around that focal length).
IMPORTANT (a trick a lot never learn) - don't take your lens down to the specimen - hold the lens right up next to your eye and bring your specimen up to it with your other hand - as it comes into focus as it gets near the lens and your eye, you will see more than you ever imagined was possible.
I mostly use the x10 lens, but if there is something tiny that I want to see in more detail (e.g. parallel striations on the face of a crystal, the "streak" of powder) I change to the x20.
These hand lenses will open up a new world to you and make identification so much easier - suddenly you can see the shape of crystals or the angles between cleavage faces, and striations. And of course colours are easier to identify on tiny samples, and you can see the colour of the powder when you scratch it with your knife blade (its "streak" - streak is often very diagnostic and can be a different colour to the colour of the mineral itself - e.g. pyrite, chalcopyrite).
You ideally want a x10 and x20 hand lense as mentioned in part 2 - cheap plastic lenses readily scratch so glass is better, lenses that fold into a cover to keep dust off and scratches away. Not too large and clunky. Some have two lenses in one cover but I prefer two separate light and small hand lenses of x10 and x20.

This one is x10 with a focal length of 18 mm (most are around that focal length).
IMPORTANT (a trick a lot never learn) - don't take your lens down to the specimen - hold the lens right up next to your eye and bring your specimen up to it with your other hand - as it comes into focus as it gets near the lens and your eye, you will see more than you ever imagined was possible.
I mostly use the x10 lens, but if there is something tiny that I want to see in more detail (e.g. parallel striations on the face of a crystal, the "streak" of powder) I change to the x20.
These hand lenses will open up a new world to you and make identification so much easier - suddenly you can see the shape of crystals or the angles between cleavage faces, and striations. And of course colours are easier to identify on tiny samples, and you can see the colour of the powder when you scratch it with your knife blade (its "streak" - streak is often very diagnostic and can be a different colour to the colour of the mineral itself - e.g. pyrite, chalcopyrite).
Series on identifying minerals - part 4 STREAK
Streak is a really important property when identifying minerals. It is the colour of the finely powdered mineral. Even though the colour of a particular mineral may vary, its streak will always be the same (so long as you always test a nice fresh piece of the mineral).

Minerals significantly softer than quartz:
For minerals softer than quartz we use a streak plate, as shown above. This is a piece of roughened or unglazed porcelain - when the mineral is rubbed on it, it leaves a coloured (or white) streak that is a characteritic of that particular mineral.
Harder minerals:
Because the hardness of porcelain is typically 6 to 6.5, minerals harder than this will not leave a streak, but will simply scratch the streak plate (so all you will see is white porcelain powder, which tells you nothing). Quartz will do this (hardness 7) and possibly even the feldspar mineral orthoclase (hardness 6), depending on the hardness of your porcelain. With these hard minerals you must crush them finely to see their streak (i.e. the colour of the powdered mineral), or scratch them with a mineral harder than them (e.g. a diamond point, a sharp bit of corundum such as sapphire).
General rules:
Coloured minerals will always produce a streak a little lighter than the true colour of the mineral.
Do not bother to test colourless or white minerals - their streak is always white.
The greatest difference between the colour and the streak of a mineral occurs with minerals that have a metallic lustre.
Silicate minerals commonly have a white streak (as well as being hard)
Minerals that produce a coloured streak are naturally easier to identify than minerals that have a white streak - it can be an important diagnostic property
Some examples: - note that the colour of minerals varies (eg hematite can also be red to blackish, pyrite can be bronzy to more silver-gray, cassiterite can be black or quite a pale colour0 - but the streak colour will be constant regardless of the mineral colour.
yellow (bronzy) pyrite produces a greenish-black streak
blackish hematite gives a red streak
black wolframite gives a brown streak
black cassiterite gives an almost colourless streak
bright yellow chalcopyrite has a greenish-black streak (more distinctly greenish than pyrite)
yellow gold has a bright shining yellow streak
pale green apatite has a white streak
green olivine has a white streak
tin-white to grey arsenopyrite has a black streak
yellow-brown to black limonite has a rusty yellow-brown streak
black tourmaline has a white streak
black magnetite has a black streak
lead-grey galena has a grey-black streak
gold-brown pyrrhotite has a grey-black streak
black chromite has a brown streak
scarlet-red cinnabar has a scarlet-red streak
copper-red native copper has a copper-red streak
So you can see that the three iron oxide minerals hematite, limonite and magnetite can be distinguished from each other (and of course magnetite is magnetic) - as can gold, pyrite and chalcopyrite - NOTHING ELSE HAS A STREAK LIKE GOLD. And combining this with other properties can be conclusine - pyrrhotite is also magnetic, gold and native copper are soft and malleable.
Streak is a really important property when identifying minerals. It is the colour of the finely powdered mineral. Even though the colour of a particular mineral may vary, its streak will always be the same (so long as you always test a nice fresh piece of the mineral).

Minerals significantly softer than quartz:
For minerals softer than quartz we use a streak plate, as shown above. This is a piece of roughened or unglazed porcelain - when the mineral is rubbed on it, it leaves a coloured (or white) streak that is a characteritic of that particular mineral.
Harder minerals:
Because the hardness of porcelain is typically 6 to 6.5, minerals harder than this will not leave a streak, but will simply scratch the streak plate (so all you will see is white porcelain powder, which tells you nothing). Quartz will do this (hardness 7) and possibly even the feldspar mineral orthoclase (hardness 6), depending on the hardness of your porcelain. With these hard minerals you must crush them finely to see their streak (i.e. the colour of the powdered mineral), or scratch them with a mineral harder than them (e.g. a diamond point, a sharp bit of corundum such as sapphire).
General rules:
Coloured minerals will always produce a streak a little lighter than the true colour of the mineral.
Do not bother to test colourless or white minerals - their streak is always white.
The greatest difference between the colour and the streak of a mineral occurs with minerals that have a metallic lustre.
Silicate minerals commonly have a white streak (as well as being hard)
Minerals that produce a coloured streak are naturally easier to identify than minerals that have a white streak - it can be an important diagnostic property
Some examples: - note that the colour of minerals varies (eg hematite can also be red to blackish, pyrite can be bronzy to more silver-gray, cassiterite can be black or quite a pale colour0 - but the streak colour will be constant regardless of the mineral colour.
yellow (bronzy) pyrite produces a greenish-black streak
blackish hematite gives a red streak
black wolframite gives a brown streak
black cassiterite gives an almost colourless streak
bright yellow chalcopyrite has a greenish-black streak (more distinctly greenish than pyrite)
yellow gold has a bright shining yellow streak
pale green apatite has a white streak
green olivine has a white streak
tin-white to grey arsenopyrite has a black streak
yellow-brown to black limonite has a rusty yellow-brown streak
black tourmaline has a white streak
black magnetite has a black streak
lead-grey galena has a grey-black streak
gold-brown pyrrhotite has a grey-black streak
black chromite has a brown streak
scarlet-red cinnabar has a scarlet-red streak
copper-red native copper has a copper-red streak
So you can see that the three iron oxide minerals hematite, limonite and magnetite can be distinguished from each other (and of course magnetite is magnetic) - as can gold, pyrite and chalcopyrite - NOTHING ELSE HAS A STREAK LIKE GOLD. And combining this with other properties can be conclusine - pyrrhotite is also magnetic, gold and native copper are soft and malleable.
Series on identifying minerals - part 5 IDENTIFICATION TABLES
There are numerous books or on-line sites which list the properties of minerals, and many are good (but a few are awful).
Hardcopy
If you want hardcopy, get a good cheap book with colour photos in it, that lists these properties of different minerals. I consider that the book "A field guide in color to Minerals, Rocks and Precious Stones by Bauer (publisher Chartwell Books Inc) is as good as any I know - it may be difficult to get at times, but is currently a real steal at $A12.70:
https://www.bookdepository.com/Fiel...-Precious-Stones-Jaroslav-Bauer/9781555210939
If you cant get it, get something that has its characteristics. It is no bigger than a small, thin paperback book but is 207 pages of tables with 576 colour photos. It groups minerals to make identification easy (e.g. "silver-white minerals of metallic lustre", 'grey minerals of metallic lustre", "yellow minerals of non-metallic lustre") and then tabulates all the properties for each mineral in each group (colour, streak, lustre, hardness, specific gravity, cleavage, physical properties, common crystal or aggregate form, occurrence, associated minerals. minerals that look similar to it, chemical formula. It lists 512 minerals (with a colour photo of each), as well as having similar photos and tables for gemstones, and for the most common rocks. It also discusses the properties used to determine minerals in more detail than I have, how to determine them, field equipment etc.
On-line
I find that the series of tables such as those by the Mineralogivcal Society of America (e.g. http://www.minsocam.org/msa/collectors_corner/id/mineral_id_keytib.htm ) are a good example of an on-line reference for mineral identification.
There are numerous books or on-line sites which list the properties of minerals, and many are good (but a few are awful).
Hardcopy
If you want hardcopy, get a good cheap book with colour photos in it, that lists these properties of different minerals. I consider that the book "A field guide in color to Minerals, Rocks and Precious Stones by Bauer (publisher Chartwell Books Inc) is as good as any I know - it may be difficult to get at times, but is currently a real steal at $A12.70:
https://www.bookdepository.com/Fiel...-Precious-Stones-Jaroslav-Bauer/9781555210939
If you cant get it, get something that has its characteristics. It is no bigger than a small, thin paperback book but is 207 pages of tables with 576 colour photos. It groups minerals to make identification easy (e.g. "silver-white minerals of metallic lustre", 'grey minerals of metallic lustre", "yellow minerals of non-metallic lustre") and then tabulates all the properties for each mineral in each group (colour, streak, lustre, hardness, specific gravity, cleavage, physical properties, common crystal or aggregate form, occurrence, associated minerals. minerals that look similar to it, chemical formula. It lists 512 minerals (with a colour photo of each), as well as having similar photos and tables for gemstones, and for the most common rocks. It also discusses the properties used to determine minerals in more detail than I have, how to determine them, field equipment etc.
On-line
I find that the series of tables such as those by the Mineralogivcal Society of America (e.g. http://www.minsocam.org/msa/collectors_corner/id/mineral_id_keytib.htm ) are a good example of an on-line reference for mineral identification.
Series on identifying minerals - part 6 MAGNETISM
Surpringly (to most people), most substances are magnetic, but only one type of magnetism is very relevant to the prospector/mineralogist (ferromagnetism).
Types of magnetism:
Diamagnetism refers to materials that are not affected by a magnetic field.
Paramagnetism refers to materials like aluminum or platinum which become magnetized in a magnetic field but their magnetism disappears when the field is removed.
Ferromagnetism refers to materials (such as iron and nickel) that can retain their magnetic properties when the magnetic field is removed.
It is ferromagnetism that is important to us for the purpose of mineral identification (ferro is Latin for iron), and it involves iron-bearing minerals (often with nickel, cobalt, titanium). The only minerals that possibly respond to magnets without heating are opaque, metallic-looking minerals.
The minerals that are magnetic range in magnetic strength from being capable of lifting steel rods to barely turning the needle on a compass. Some minerals may not be magnetic, but are still attracted to magnets, and it is important to distinguish these two types as either magnetic (e.g. magnetite, some pyrrhotite)

- or as attracted to magnets - most identification tables dont make this distinction.
Magnetism is an unreliable property as not all specimens of some minerals will demonstrate it (e.g. pyrrhotite is a mineral that varies a bit in its iron content, and only iron-rich varieties are noticeably magnetic).
Minerals magnetic, or attracted to (or repelled by) magnets
Babingtonite (weak)
Bismuth, i.e. native bismuth (the only mineral very weakly REPELLED, not attracted - diamagnetic)
Chromite (weak)
Columbite (weak)
Ferberite (weak)
Franklinite (weak, paramagnetic)
Hematite (very weak, paramagnetic)
Ilmenite (weak, always when heated)
Iron-nickel e.g. as in some meteorites (attracted to magnets)
Magnetite (strong and consistent, ferromagnetic) the only natural magnet itself, hence its name (i.e. it will attract things like iron filings and small iron naila)
Maghemite (strong)
Manganbabingtonite (very weak)
Platinum (weak, not strictly paramagnetic but the common iron inclusions in it are)
Pyrrhotite (sometimes strong, but is inconsistent - ferromagnetic)
Siderite (weak but only when heated)
Tantalite (weak)
Most beginners or those with only modest magnets will only distinguish magnetite, iron-nickel alloy, maghemite or pyrrhotite as magnetic they are often to always distinctive.
Equipment for testing magnetism
A tiny compass is probably the most sensitive indicator of magnetism the needle will deflect when brought close to the mineral. Only use a 50 cent variety the compass will be permanently wrecked for any use in navigation (its needle will become magnetised increasingly with time). For this reason I dont use a compass in practice.
The most effective testing results are obtained with the use of a powerful magnet (unfortunately with common magnets this is a function of size, and no-one wants a large horseshoe magnet in their pocket wrecking their credit cards and compass). However it is also a function of the alloy material the magnet is made of. In practice I use a bar magnet (a rod) dangling on a bit of string or leather boot-lace and hanging from the same leather thong (necklace) that has my hand lenses and tiny pocket knife. Prospectors Supplies sell these bar magnets but I have cut two-thirds of the plastic-rod part of its length off to shorten it. I keep a tiny bit of rough ceramic in my pocket for determining streak (along with the knife point), together with a bit of quartz and a gold coin to assist with hardness-testing (also along with the knife point and my fingernail). I have a tiny plastic bottle (with plastic screw-top) of 50% hydrochloric acid (muriatic acid) in my field bag to test carbonate minerals, but I keep it in a thick plastic sandwich bag as well, two usually with one inside the other (otherwise drips will soon eat a hole in your bag).
To test for magnetism, hold the specimen in one hand and bring the end of the magnet towards it - if the mineral you are testing is magnetic the magnet will be pulled sideways towards the grain you are testing, i.e. it will sway slightly (because it is dangling from string it is a very sensitive test). That way you can even test small grains within a single larger lump of a different mineral, or within a rock.
Remember to combine this test with other properties - the three iron minerals magnetite, hematite and limonite have different streak colours, you will never detect attraction of pure hematite towards a magnet with your simple equipment, the fourth common iron mineral maghemite is magnetic and has a reddish streak similar to hematite but not the black streak colour of magnetite.
Surpringly (to most people), most substances are magnetic, but only one type of magnetism is very relevant to the prospector/mineralogist (ferromagnetism).
Types of magnetism:
Diamagnetism refers to materials that are not affected by a magnetic field.
Paramagnetism refers to materials like aluminum or platinum which become magnetized in a magnetic field but their magnetism disappears when the field is removed.
Ferromagnetism refers to materials (such as iron and nickel) that can retain their magnetic properties when the magnetic field is removed.
It is ferromagnetism that is important to us for the purpose of mineral identification (ferro is Latin for iron), and it involves iron-bearing minerals (often with nickel, cobalt, titanium). The only minerals that possibly respond to magnets without heating are opaque, metallic-looking minerals.
The minerals that are magnetic range in magnetic strength from being capable of lifting steel rods to barely turning the needle on a compass. Some minerals may not be magnetic, but are still attracted to magnets, and it is important to distinguish these two types as either magnetic (e.g. magnetite, some pyrrhotite)

- or as attracted to magnets - most identification tables dont make this distinction.
Magnetism is an unreliable property as not all specimens of some minerals will demonstrate it (e.g. pyrrhotite is a mineral that varies a bit in its iron content, and only iron-rich varieties are noticeably magnetic).
Minerals magnetic, or attracted to (or repelled by) magnets
Babingtonite (weak)
Bismuth, i.e. native bismuth (the only mineral very weakly REPELLED, not attracted - diamagnetic)
Chromite (weak)
Columbite (weak)
Ferberite (weak)
Franklinite (weak, paramagnetic)
Hematite (very weak, paramagnetic)
Ilmenite (weak, always when heated)
Iron-nickel e.g. as in some meteorites (attracted to magnets)
Magnetite (strong and consistent, ferromagnetic) the only natural magnet itself, hence its name (i.e. it will attract things like iron filings and small iron naila)
Maghemite (strong)
Manganbabingtonite (very weak)
Platinum (weak, not strictly paramagnetic but the common iron inclusions in it are)
Pyrrhotite (sometimes strong, but is inconsistent - ferromagnetic)
Siderite (weak but only when heated)
Tantalite (weak)
Most beginners or those with only modest magnets will only distinguish magnetite, iron-nickel alloy, maghemite or pyrrhotite as magnetic they are often to always distinctive.
Equipment for testing magnetism
A tiny compass is probably the most sensitive indicator of magnetism the needle will deflect when brought close to the mineral. Only use a 50 cent variety the compass will be permanently wrecked for any use in navigation (its needle will become magnetised increasingly with time). For this reason I dont use a compass in practice.
The most effective testing results are obtained with the use of a powerful magnet (unfortunately with common magnets this is a function of size, and no-one wants a large horseshoe magnet in their pocket wrecking their credit cards and compass). However it is also a function of the alloy material the magnet is made of. In practice I use a bar magnet (a rod) dangling on a bit of string or leather boot-lace and hanging from the same leather thong (necklace) that has my hand lenses and tiny pocket knife. Prospectors Supplies sell these bar magnets but I have cut two-thirds of the plastic-rod part of its length off to shorten it. I keep a tiny bit of rough ceramic in my pocket for determining streak (along with the knife point), together with a bit of quartz and a gold coin to assist with hardness-testing (also along with the knife point and my fingernail). I have a tiny plastic bottle (with plastic screw-top) of 50% hydrochloric acid (muriatic acid) in my field bag to test carbonate minerals, but I keep it in a thick plastic sandwich bag as well, two usually with one inside the other (otherwise drips will soon eat a hole in your bag).
To test for magnetism, hold the specimen in one hand and bring the end of the magnet towards it - if the mineral you are testing is magnetic the magnet will be pulled sideways towards the grain you are testing, i.e. it will sway slightly (because it is dangling from string it is a very sensitive test). That way you can even test small grains within a single larger lump of a different mineral, or within a rock.
Remember to combine this test with other properties - the three iron minerals magnetite, hematite and limonite have different streak colours, you will never detect attraction of pure hematite towards a magnet with your simple equipment, the fourth common iron mineral maghemite is magnetic and has a reddish streak similar to hematite but not the black streak colour of magnetite.
malri_au
Steve
just like to add something that has helped me in the past.
'Field Tests For The Common Mineral Elements'
http://www.webpal.org/SAFE/aaarecovery/5_simple_technology/basics/UofA Field Tests.pdf
'Field Tests For The Common Mineral Elements'
http://www.webpal.org/SAFE/aaarecovery/5_simple_technology/basics/UofA Field Tests.pdf
Series on identifying minerals - part 7 FLAME AND BEAD TESTS
Thanks. Yes, these are often effective. We used to use these tests when I was studying geology in the 1960s but they are still quite useful. However I will mostly focus my series on tests using what people carry in their pockets. on their lanyard, or in their day-pack. If you are a keen mineral collector and identifier, it would be worth putting this together (I still have my platinum wire in a glass-rod handle, but have not used it in 40 years, the reason being that if it is important enough to me I get a chemical analysis or XRay powder photo nowadays - but these are relatively expensive unless you have many samples to analyse - I have access to this equipment, including a microprobe analyser where I can analyse things fully down to a few tens of microns in size).
Still, it does not require a lot to determine many elements:
https://www.thoughtco.com/bead-test-in-chemical-analysis-4050801
https://www.thoughtco.com/how-flame-test-colors-are-produced-3963973
Things like blowpipe analysis can give off toxic fumes that were not fully appreciated in those days, so make sure the draught or breeze is blowing the other way and use tiny amounts.
Flame tests (powder in a candle flame) are safe and fast and can be useful:
https://www.thoughtco.com/how-to-do-the-flame-test-3976094
Flame Colors of Metals
magenta: lithium
lilac: potassium
azure blue: selenium
blue: arsenic, cesium, copper(I), indium, lead
blue-green: copper(II) halide, zinc
pale blue-green: phosphorus
green: copper(II) non-halide, thallium
bright green: boron
pale to apple green: barium
pale green: antimony, tellurium
yellowish green: manganese(II), molybdenum
intense yellow: sodium
gold: iron
orange to red: calcium
red: rubidium
crimson: strontium
bright white: magnesium
They have limitations (eg most minerals contain multiple metals and one element can color a flame so strongly that the color of another element is masked). However if you have tried the other physical tests that I mention in this blog above, you usually know what a mineral is likely to be and it may confirm it. However you can see the issue with many shades of green etc. However transparent colourless heavy crystals might show as cerrussite or anglesite (blue flame for lead), not barite (pale green flame for barium). Also sometimes silicate minerals will be identified if pure rather than mixtures (eg the feldspars which are K, Na or Ca if pure, but are often mixtures - similarly many dark minerals contain iron and/or magnesium with or without calcium, sodium). Black tourmaline may look like cassiterite but will give the bright green of boron.
malri_au said:just like to add something that has helped me in the past.
'Field Tests For The Common Mineral Elements'
http://www.webpal.org/SAFE/aaarecovery/5_simple_technology/basics/UofA Field Tests.pdf
Thanks. Yes, these are often effective. We used to use these tests when I was studying geology in the 1960s but they are still quite useful. However I will mostly focus my series on tests using what people carry in their pockets. on their lanyard, or in their day-pack. If you are a keen mineral collector and identifier, it would be worth putting this together (I still have my platinum wire in a glass-rod handle, but have not used it in 40 years, the reason being that if it is important enough to me I get a chemical analysis or XRay powder photo nowadays - but these are relatively expensive unless you have many samples to analyse - I have access to this equipment, including a microprobe analyser where I can analyse things fully down to a few tens of microns in size).
Still, it does not require a lot to determine many elements:
https://www.thoughtco.com/bead-test-in-chemical-analysis-4050801
https://www.thoughtco.com/how-flame-test-colors-are-produced-3963973
Things like blowpipe analysis can give off toxic fumes that were not fully appreciated in those days, so make sure the draught or breeze is blowing the other way and use tiny amounts.
Flame tests (powder in a candle flame) are safe and fast and can be useful:
https://www.thoughtco.com/how-to-do-the-flame-test-3976094
Flame Colors of Metals
magenta: lithium
lilac: potassium
azure blue: selenium
blue: arsenic, cesium, copper(I), indium, lead
blue-green: copper(II) halide, zinc
pale blue-green: phosphorus
green: copper(II) non-halide, thallium
bright green: boron
pale to apple green: barium
pale green: antimony, tellurium
yellowish green: manganese(II), molybdenum
intense yellow: sodium
gold: iron
orange to red: calcium
red: rubidium
crimson: strontium
bright white: magnesium
They have limitations (eg most minerals contain multiple metals and one element can color a flame so strongly that the color of another element is masked). However if you have tried the other physical tests that I mention in this blog above, you usually know what a mineral is likely to be and it may confirm it. However you can see the issue with many shades of green etc. However transparent colourless heavy crystals might show as cerrussite or anglesite (blue flame for lead), not barite (pale green flame for barium). Also sometimes silicate minerals will be identified if pure rather than mixtures (eg the feldspars which are K, Na or Ca if pure, but are often mixtures - similarly many dark minerals contain iron and/or magnesium with or without calcium, sodium). Black tourmaline may look like cassiterite but will give the bright green of boron.
Series on identifying minerals - part 7 FLAME AND BEAD TESTS
Thanks. Yes, these are often effective. We used to use these tests when I was studying geology in the 1960s but they are still quite useful. However I will mostly focus my series on tests using what people carry in their pockets. on their lanyard, or in their day-pack. If you are a keen mineral collector and identifier, it would be worth putting this together (I still have my platinum wire in a glass-rod handle, but have not used it in 40 years, the reason being that if it is important enough to me I get a chemical analysis or XRay powder photo nowadays - but these are relatively expensive unless you have many samples to analyse - I have access to this equipment, including a microprobe analyser where I can analyse things fully down to a few tens of microns in size).
Still, it does not require a lot to determine many elements:
https://www.thoughtco.com/bead-test-in-chemical-analysis-4050801
https://www.thoughtco.com/how-flame-test-colors-are-produced-3963973
Things like blowpipe analysis can give off toxic fumes that were not fully appreciated in those days, so make sure the draught or breeze is blowing the other way and use tiny amounts.
Flame tests (powder in a candle flame) are safe and fast and can be useful:
https://www.thoughtco.com/how-to-do-the-flame-test-3976094
Flame Colors of Metals
magenta: lithium
lilac: potassium
azure blue: selenium
blue: arsenic, cesium, copper(I), indium, lead
blue-green: copper(II) halide, zinc
pale blue-green: phosphorus
green: copper(II) non-halide, thallium
bright green: boron
pale to apple green: barium
pale green: antimony, tellurium
yellowish green: manganese(II), molybdenum
intense yellow: sodium
gold: iron
orange to red: calcium
red: rubidium
crimson: strontium
bright white: magnesium
They have limitations (eg most minerals contain multiple metals and one element can color a flame so strongly that the color of another element is masked). However if you have tried the other physical tests that I mention in this blog above, you usually know what a mineral is likely to be and it may confirm it. However you can see the issue with many shades of green etc. However transparent colourless heavy crystals might show as cerrussite or anglesite (blue flame for lead), not barite (pale green flame for barium). Also sometimes silicate minerals will be identified if pure rather than mixtures (eg the feldspars which are potassium, sodium or calcium if pure, but are often mixtures - similarly many dark minerals contain iron and/or magnesium with or without calcium, sodium). Black tourmaline may look like cassiterite but will give the bright green of boron.
malri_au said:just like to add something that has helped me in the past.
'Field Tests For The Common Mineral Elements'
http://www.webpal.org/SAFE/aaarecovery/5_simple_technology/basics/UofA Field Tests.pdf
Thanks. Yes, these are often effective. We used to use these tests when I was studying geology in the 1960s but they are still quite useful. However I will mostly focus my series on tests using what people carry in their pockets. on their lanyard, or in their day-pack. If you are a keen mineral collector and identifier, it would be worth putting this together (I still have my platinum wire in a glass-rod handle, but have not used it in 40 years, the reason being that if it is important enough to me I get a chemical analysis or XRay powder photo nowadays - but these are relatively expensive unless you have many samples to analyse - I have access to this equipment, including a microprobe analyser where I can analyse things fully down to a few tens of microns in size).
Still, it does not require a lot to determine many elements:
https://www.thoughtco.com/bead-test-in-chemical-analysis-4050801
https://www.thoughtco.com/how-flame-test-colors-are-produced-3963973
Things like blowpipe analysis can give off toxic fumes that were not fully appreciated in those days, so make sure the draught or breeze is blowing the other way and use tiny amounts.
Flame tests (powder in a candle flame) are safe and fast and can be useful:
https://www.thoughtco.com/how-to-do-the-flame-test-3976094
Flame Colors of Metals
magenta: lithium
lilac: potassium
azure blue: selenium
blue: arsenic, cesium, copper(I), indium, lead
blue-green: copper(II) halide, zinc
pale blue-green: phosphorus
green: copper(II) non-halide, thallium
bright green: boron
pale to apple green: barium
pale green: antimony, tellurium
yellowish green: manganese(II), molybdenum
intense yellow: sodium
gold: iron
orange to red: calcium
red: rubidium
crimson: strontium
bright white: magnesium
They have limitations (eg most minerals contain multiple metals and one element can color a flame so strongly that the color of another element is masked). However if you have tried the other physical tests that I mention in this blog above, you usually know what a mineral is likely to be and it may confirm it. However you can see the issue with many shades of green etc. However transparent colourless heavy crystals might show as cerrussite or anglesite (blue flame for lead), not barite (pale green flame for barium). Also sometimes silicate minerals will be identified if pure rather than mixtures (eg the feldspars which are potassium, sodium or calcium if pure, but are often mixtures - similarly many dark minerals contain iron and/or magnesium with or without calcium, sodium). Black tourmaline may look like cassiterite but will give the bright green of boron.
Syndyne
Shaun Galman
Fantastic thread ideas. Thanks for taking the time to post this stuff up Goldierocks :Y:
Cheers,
Shauno.
Cheers,
Shauno.
malri_au said:nvm,why bother eh?
Why do anything? People not interested in doing anything probably don't post on this site
If you mean why do chemical tests, then yes, it usually isn't necessary once you get used to other methods of mineral identification. Why collect minerals? I have one mate with a collection valued at more than $2 million now, another more than $1 million, and it is a billion dollar trade.... But most just enjoy doing it. I have had single specimens a centimetre or two in size worth thousands.
StoneTheCrows
Neil Mulvaney
Yep
More good reading and learning so thank you..
More good reading and learning so thank you..
Note that identifying your own minerals is under another thread - "Your mineral identification questions". My idea was to give the basic principles here, and that people then put all their questions under the other thread. I can only drop in occassionally. but once people get the hang of it they can assist each other - I alrady see people giving more details of their unknown minerals on the other thread, so it may be working - and there are others there who know their minerals already and who are assisting with comments.
malri_au
Steve
What I meant was,flame tests.
You pretty much validate that with what you posted after.
Now,chemical tests are another,Stannous Chloride for gold being the most important to me.
Depending on what you are chasing,chemical or flame tests are just as viable as other methods.
Safety is always an issue,you shouldn't have to tell people fire is hot though.
You pretty much validate that with what you posted after.
Now,chemical tests are another,Stannous Chloride for gold being the most important to me.
Depending on what you are chasing,chemical or flame tests are just as viable as other methods.
Safety is always an issue,you shouldn't have to tell people fire is hot though.
Similar threads
- Replies
- 23
- Views
- 1K
- Replies
- 2
- Views
- 1K
- Replies
- 2
- Views
- 1K


