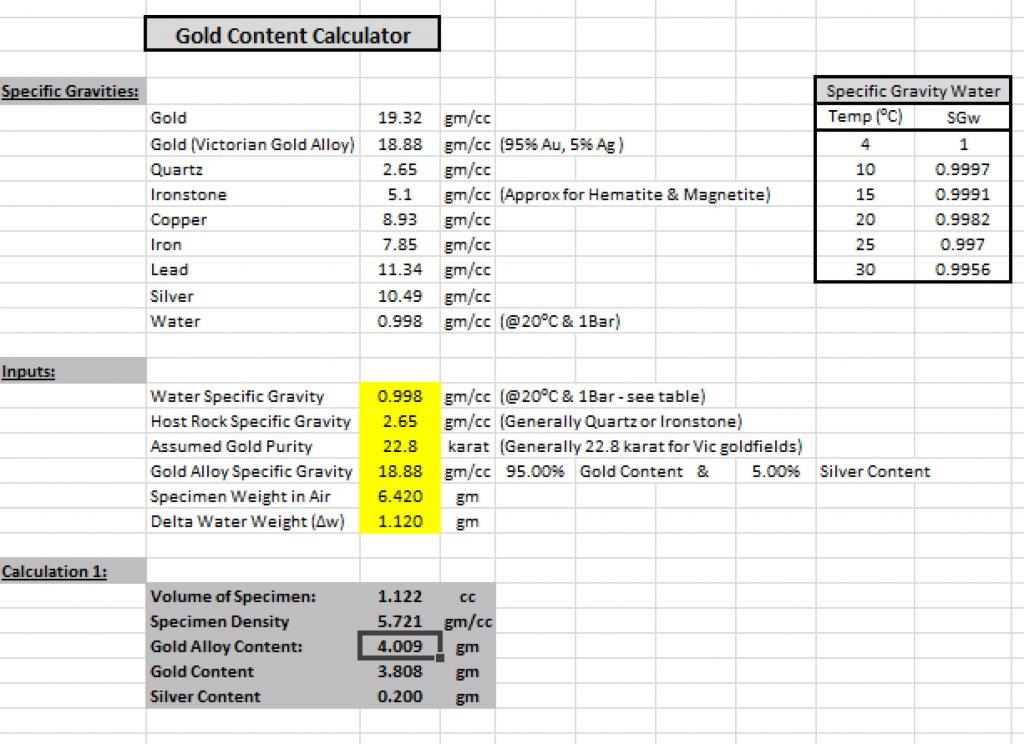
Colin, I use the xcel based spread sheet program as shown below. It only works where the host rock is not a combination such as quartz and ironstone.
The inputs are the cells shaded yellow.
1. The water specific gravity is entered from the top right table depending on water temperature.
2. The host rock's specific gravity is then entered. If it is a combination of dissimilar material such as quartz and ironstone, this method will provide Specific Gravity, but not the gold content.
3. Next enter the Gold purity in karats. It's generally around 22.8 karat (for the GT - 95%AU & 5%Ag), but may vary greatly from field to field. Might have to do some research for your specific goldfield.
4. The next yellow cell calculates the gold alloy Specific Gravity assuming Silver accounts for most of the alloying material. There's Cu in the GT gold, but generally pretty small.
5. You then measure the specimen's dry weight (on scales as you normally would).
6. Now measure the volume of the specimen. This is done by placing a small plastic tub of water on your scales (just big enough to immerse your specimen in), and zeroing the scales (using tare button) to 0.00g. Now with the specimen held on a small cotton thread. Immerse it in the water jar. And note the weight increase on the scales. As water has a Specific Gravity very close to 1.0g/cc, if the weight goes up by 1.5g say, then the volume is thus 1.5cc. There is a very small correction based on the temperature of water (used in step 1). Make sure there is no air trapped on the sample when immersed in the water.
7. The Specific Gravity of the specimen is then simply calculated by dividing the dry weight by the measured volume.
8. Some fairly simple math is then used to determine what ratio of gold alloy to the host material would equal that specimen's SG, then using the known volume, calculate the gold alloy content.
Clearly there are a number of assumptions involved (including gold alloy purity), but the results should be +/- say 5%
I can email the spread sheet to you if required.