Hi Folks,
Found a gold specimen yesterday and decided to do a specific gravity test to determine the approximate gold content. Thought I'd share this task with you as I performed the test and calculations.
There is quite a bit on the net about this process and there appears to be a few variations on how this operation could be performed. After looking what was involved, I decided to use an approach that was as simple as possible. I ended up using information from a couple of sources to keep the whole operation a short and uncomplicated task. The test was based on a specimen that contained gold and quartz.
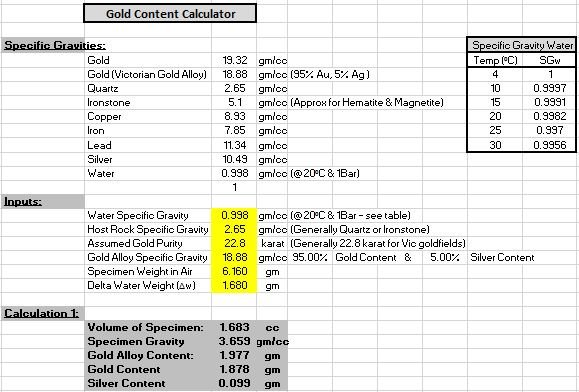
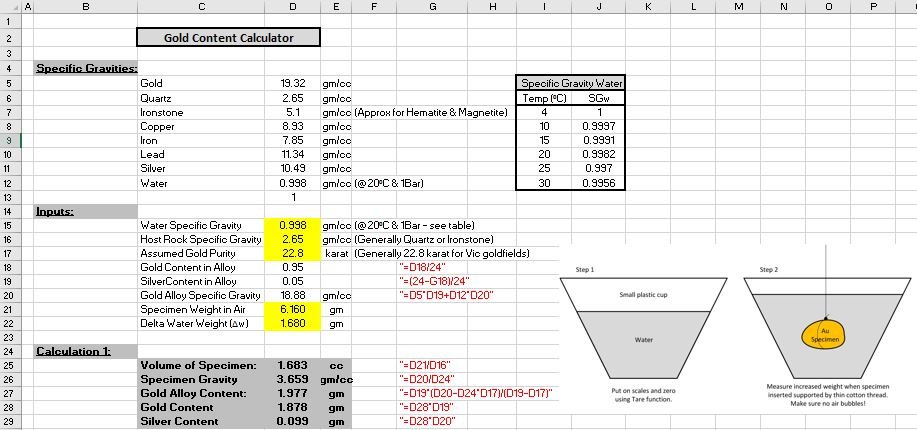
Step one was to find the specific gravity of the sample. Gold has a SG of about 19.3 (19.3 times as heavy as water for the same volume) and quartz has a SG of about 2.65. The sample was weighed on a set of scales

A small container of water was then placed on the scales and then the Tar button was pressed to zero the scales. A piece of thin cotton was tied to the specimen which was then suspended in the water. I held the piece I so that it did not touch the bottom or sides of the container. The level of the water increased in the container equal to the volume of the specimen. The weight shown ( in grams) is also equal to the volume of the gold specimen (in cubic centimetres)

To find the specific gravity of the sample piece, the dry weight is divided by the volume. In my case: 6.16 /1.68 = 3.667
I then used a chart taken from Gold Prospecting WA
To calculate the percentage of gold in the sample. Using the table, I found the specific gravity that was closest to my sample (left hand column) and then was able to look up the approximate % of gold in the quartz. (Right hand column)
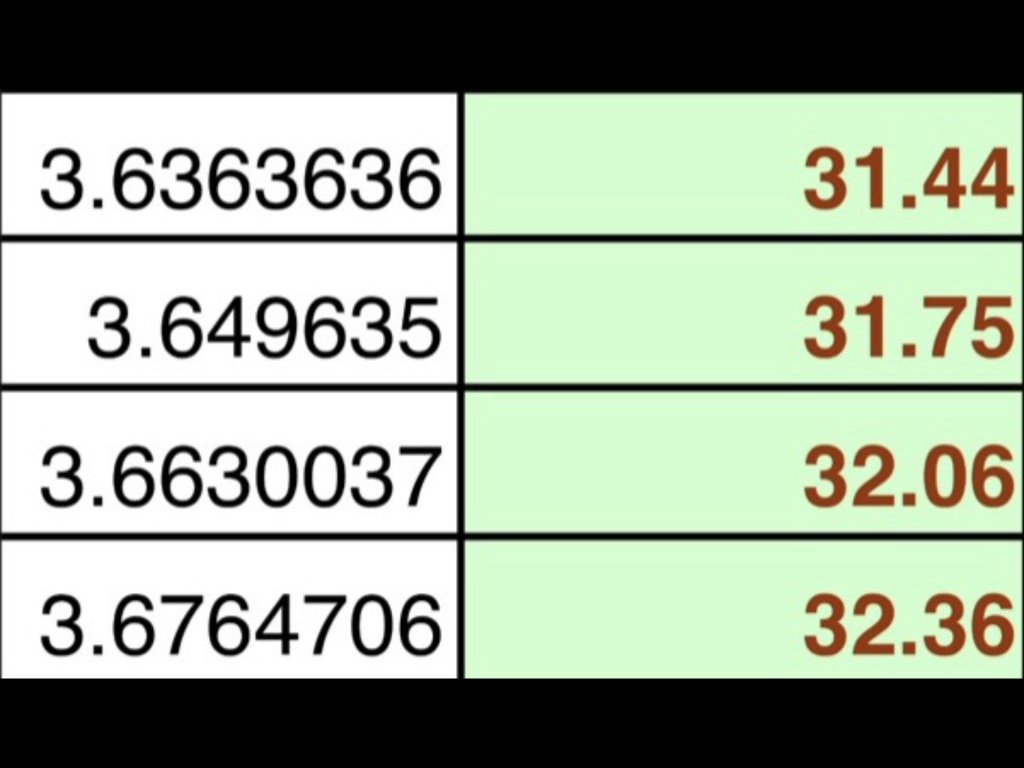
This takes less time than manually performing the calculations required. As you can see from the segment of the table, the percentage of gold in my specimen is approximately 32.06. (Actually a little more than what was in the table but the difference is only minimal) The actual weight of gold is then calculated as 32.06% of the specie dry weight.
(.3206 x 6.16 = 1.975g) Obviously this is an approximation and there may be some error due to impurities in both the gold and quartz but it does give a reasonable indication the gold weight.
Hope this is of some help to others wanting to perform a similar calculation.
Cheers
Les
Found a gold specimen yesterday and decided to do a specific gravity test to determine the approximate gold content. Thought I'd share this task with you as I performed the test and calculations.
There is quite a bit on the net about this process and there appears to be a few variations on how this operation could be performed. After looking what was involved, I decided to use an approach that was as simple as possible. I ended up using information from a couple of sources to keep the whole operation a short and uncomplicated task. The test was based on a specimen that contained gold and quartz.
Step one was to find the specific gravity of the sample. Gold has a SG of about 19.3 (19.3 times as heavy as water for the same volume) and quartz has a SG of about 2.65. The sample was weighed on a set of scales

A small container of water was then placed on the scales and then the Tar button was pressed to zero the scales. A piece of thin cotton was tied to the specimen which was then suspended in the water. I held the piece I so that it did not touch the bottom or sides of the container. The level of the water increased in the container equal to the volume of the specimen. The weight shown ( in grams) is also equal to the volume of the gold specimen (in cubic centimetres)

To find the specific gravity of the sample piece, the dry weight is divided by the volume. In my case: 6.16 /1.68 = 3.667
I then used a chart taken from Gold Prospecting WA
To calculate the percentage of gold in the sample. Using the table, I found the specific gravity that was closest to my sample (left hand column) and then was able to look up the approximate % of gold in the quartz. (Right hand column)

This takes less time than manually performing the calculations required. As you can see from the segment of the table, the percentage of gold in my specimen is approximately 32.06. (Actually a little more than what was in the table but the difference is only minimal) The actual weight of gold is then calculated as 32.06% of the specie dry weight.
(.3206 x 6.16 = 1.975g) Obviously this is an approximation and there may be some error due to impurities in both the gold and quartz but it does give a reasonable indication the gold weight.
Hope this is of some help to others wanting to perform a similar calculation.
Cheers
Les